Abstract
Tomato early blight caused by Alternaria solani is an economically important disease causing threat to tomato cultivation wherever it’s grown. This study evaluated the effect of three different plant extracts Neem (Azadirachta indica), Garlic (Allium sativa) and Ginger (Zingiber officinale) against Alternaria solani the causative agent of early blight of tomato. The experiment was 3 × 5 factorial pitted in a Completely Randomized Design (CRD). The treatment were garlic, ginger and neem extracts at four different concentration (25%, 50%, 75% and 100%) control and check which were replicated three times. Data were recorded on mycelial growth of the fungus and percent growth inhibition of the extracts against A. solani. The result from the study revealed that neem extract had the least mycelial growth as well as higher percent growth inhibition compared to other treatments, followed by garlic throughout the inoculation periods. Plant extracts at 75% and 100% had the least mycelial growth compared to the control. Plant extracts at lower concentration of 25 and 50% exhibited the least percent growth inhibition compared to the control. Neem and garlic extracts at 75% and 100% concentration significantly inhibited the growth of the fungus and could therefore be recommended for further studies in the screen house and field to evaluate their efficacies against early blight of tomato under field condition.
Keywords: Plant extract, mycelial growth, mycelial growth inhibition, neem, garlic, ginger
INTRODUCTION
The cultivated tomato (Solanum lycopersicum L.) is the world’s most highly consumed vegetable due to its status as a basic ingredient in a large variety of raw, cooked or processed foods (FAOSTAT, 2023). It belongs to the family Solanaceae, which includes several other commercially important species. Tomato is grown worldwide for local use or as an export crop (FAOSTAT, 2023).The crop is a traditional vegetable crop commercially cultivated throughout the world on 4.02 million hectares with a production of 152.9 million tones and productivity of 37.8 tones/ha. Fresh tomato production reached 163,719,357 tons in the world in 2023, out of which about 4.5% are traded (FAOSTAT, 2023).
The commercially important tomato fruit can vary in color, size and shape (Vaughan and Geissler, 1997). The fruit contains a large quantity of water, vitamins and minerals, low amounts of proteins and fats, and some carbohydrates. It also contains carotenes, such as lycopene (which gives the fruit its predominantly red color) and beta-Carotene which gives the fruit its orange color (OECD, 2008). Modern tomato cultivars produce fruits that contain up to 3% sugar of fresh fruit weight. It also contains tomatine, an alkaloid with fungicidal properties (OECD, 2008).
There are many vegetable fruits recognized in Nigeria, but tomato as a vegetable fruit is a major food component. Tomato has been in cultivation in Nigeria for a very long time and it is an important component of the daily diet, consumed both fresh and in paste form (Taofiq, 2017). It is an ingredient utilized by every household and constitutes the national food security programme. Nigeria is currently the second largest producer of fresh tomatoes in Africa (Taofiq, 2017) producing 10.8% of fresh tomatoes in the region. Globally, Nigeria is the 14th largest tomato producer with 2.3 million tones (FAOSTAT, 2023).
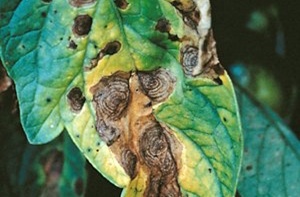
Though Nigeria is the second largest producer of tomato in Africa, yet the production is hampered by biotic and abiotic factors. Among the major constraint to production in Nigeria are insect pests and diseases, rainfall, lack of quality seeds, weeds, environmental factors, socio- economic factors, etc. One of the diseases of economic importance in tomato production which causes high losses in tomato fruits is early leaf blight of tomato. Early leaf blight (Alternaria tomatophila and A. solani) also known as Alternaria leaf blight is a widespread disease in which the older, lower leaves are the common initial site of infection as they are more susceptible. Lesions begin as small, dark, irregularly shaped spots that progress into increasingly larger, concentric rings. As the disease develops, it can cause leaf death and reduced yields. Although infection often begins in the lower portion of the plant, it can extend into the upper leaves, and stems and fruits can be infected at any stage. Stem infection can progress into Alternaria stem canker (Chohan et al., 2015).
Several attempts have been made to control early blight of tomato through cultural, physical, chemical, and biological methods (Haruna et al., 2024). The early blight fungi of tomato have very large host range hence cultural control methods have been used and are the most widely acceptable means of controlling the disease as one species has many hosts. However, cultural control methods may not sufficiently control the diseases, there is a need to integrate it with other control methods that are equally safe to consumers and the environment. Hence, there a need to evaluate some plant extracts in vitro on mycelial growth and percent growth inhibition of the A. solani, the causal agent of tomato early blight.
MATERIALS AND METHODS
Experimental site
The laboratory experiment was conducted in the teaching and research laboratory of the Department of Crop protection, Faculty of Agriculture, Bayero University Kano. Diseased tomato fruits were collected from the Center for Dryland Agriculture Orchard, Bayero University with coordinates latitude 11.58 ºN and longitude 8.25º E and taken to the laboratory for further work.
Isolation of the organism
Fruits of tomato showing symptoms of early blight collected from the orchard were sorted out, and attached sands and dirt removed from them. A small section of an advancing margins of the lesion was cut with a sterilized pair of scissors. The samples were then sterilized with 1 % sodium hypochlorite (NaClO) for 3 minutes and then rinsed with distilled water three times and dried with sterile tissue (Larone, 1995). The samples were aseptically transferred into Petri dishes containing 15 mL of molten Potato Dextrose Agar (PDA) amended with streptomycin to prevent growth of bacterial and other unwanted microorganisms. The plates were incubated for 7 days at room temperature (27-30 °C). Distinct colony present on the plate was sub cultured for another 7 days to get pure culture and for further growth. The isolated organism was stored in an agar slant for the in vitro experiment.
Identification of the organism (Alternaria solani)
To identify the fungi, a drop of lactophenol cotton blue stain was placed in a clean slide with the aid of a mounted needle, a small portion of the mycelium from the fungal cultures was removed and placed in the drop of the stain. The mycelium was spread thoroughly on the slide with the aid of two sterilized needles and cover the slide. The covered slide was examined under the microscope as described by (Fawole and Oso, 1995; Muhammad et al., 2018). The observation was done at high power resolution of the microscope (×40) for clarity. The cultures observed were identified by microscopic and morphological examinations using identification manual (Barnett and Hunter, 2006).
Collection of plant material and preparation of plant extracts
Three plant materials Ginger (Zingiber officinale), Garlic (Allium sativum), Neem (Azadirachta indica) were used in this experiment. The plant materials (ginger and garlic) were purchased from Rimi market in Kano state, while neem was obtained from the premises of faculty of Agriculture, Bayero University Kano. The plant materials were rinsed three times with tap water and then washed with distilled water, air dry under shade. The plant extracts were grinded using blender (Binatone Multi pupose blender) into powder and kept for further use in-vitro experiment by incorporating it into the PDA. Ten grams (10 g) of each of the plant materials was used for the experiment.
Preparation of media and the stock solution of the plant extracts
Preparation of stock solution
Ten grams (10 g) of the powder from each of the plant was added to 200 mL of distilled water in a 1000 mL flat bottom flask, as stock solution and the suspension was allowed to stand overnight and the concentration filtered using muslin cloth and then kept in a glass bottle. The extract at different concentration were prepared by mixing 25 mL, 50 mL, 75 mL and 100 mL of stock solution with 100 mL of distilled water to get final concentration of extract at 25 %, 50 %, 75% and 100 % respectively.
Culture media preparation
200 g of peeled potato was boiled and filtered with muslin cloth, 20 g of dextrose and 18 g of agar were weighed and added to 1000 mL of sterile distilled water. The medium was then sterilized by autoclaving at 121°C at 15 Ibs pressure for 15 minutes, after which it was removed and allowed to cool down (45-50 oC). The PDA was amended with the plant extracts at different concentrations and poured aseptically to the Petri dishes and shake for even distribution of the extract into the media and allowed to solidify. Prior to the pouring of the media, it was supplemented with streptomycin (10 μg/mL), to check bacterial growth.
In vitro Assessment of antifungal potential of the plant extracts
A mycelia plugs (5 mm in diameter) were taken from a week old revived A. solani and placed at the centre of the petri dishes containing the plant extracts amended PDAs.The in-vitro experiment were laid in a Complete randomized design (CRD) consisting of 3 treatment replicated 3 times. Garlic extract labelled as T1, Neem extract as T2, Ginger extract as T3, Synthetic fungicide (Ridomil Gold) Check as T4 and Control as T5, Control had no extracts and no synthetic fungicide. The mycelial growth diameter of the pathogen was measured using meter ruler. Fungal growth was measured in each of the treatments including control 2-8 days post-inoculation periods. Percentage reduction on mycelia or zone inhibition was determined according to the formula,
% Inhibition = C-T/C * 100 (Lurwanu et al., 2021)
Where: C = Mycelial growth of fungus in control
T = Mycelial growth of fungus in treatment
Data collection and data analysis
The data on mycelia growth and mycelial growth inhibition from each treatment were taken and subjected to analysis of variance (ANOVA) using GENSTAT software. Means were separated using Student-Newman-Keuls (SNK) at 5 % level of significance.
RESULTS
Identification of the organism (Alternaria solani)
The fungal pathogen identified using microscopic examination showed a typical morphology of Alternaria spp. The fungus identified was believed to be the causal pathogen of tomato early blight as shown in plate 1.
Effect of the plant extracts concentration on mycelial growth of Alternaria solani at 2 to 8 days after inoculation (DAI)
The result of in-vitro experiment in Table 1 shows the effect of plant extracts on mycelial growth of Alternaria solani at 2,4,6, and 8 days after inoculation. The result of the in-vitro plant extract on mycelial growth of A. solani shows no significant difference between the plant extracts at 2 days. At 4 to 8 days after inoculation, ginger extract had the highest mycelial growth and varied with the other extracts statistically. While, Neem extract had the lowest mycelial growth value followed by Garlic at 4 to 8 DAI. The table also shows the effect level of concentration of the plant extracts at which Alternaria solani was inoculated which are 25%, 50%, 75% 100%, Ridomil gold (as Check) and 0% which is the control. Second to the highest and highest concentration (75 % and 100 %) had the least mycelial growth compared to the control and check, and were found to be very effective in suppressing the growth of A. solani.
Significant differences were observed in the interaction between the plant extracts and the various concentration used at all the inoculation days (Table 1).
Interaction between plant extract and their different concentrations on the mycelial growth of A. solani at 2, 4, 6 and 8 days after inoculation
Significant interaction between the plant extracts and the different concentration used at all the inoculation periods (2-8 DAI) were highly significant. The lowest concentration of 25 and 50% recorded highest mycelial growth, whereas the highest concentrations of 75 and 100% recorded the lowest mycelial growth compared to control (Table 2, 3, 4 and 5).
Effect of plant extracts at different concentration on percent growth inhibition of Alternaria solani 2 - 8 days after inoculation
The result in Table 6 showed the percent inhibition growth of Alternaria solani. The results indicates that Neem at 4, 6, and 8 day after inoculation had higher percent inhibition followed by Garlic. Ginger had the least percent inhibition compared to control (Table 6).The table also shows the results of different levels of concentration of the plant extracted used in the experiment. The highest concentrations 100 % and 75 % are more effective in percent inhibition compared to 0 % and 25 % level of concentration compared to control (Table 6).
Interaction effects between plant extract and their different concentrations on the growth percentage inhibition of A. solani at 2-8 days after inoculation
The interaction between the plant extracts and the different concentration used at all the inoculation periods (2-8 DAI) were highly significant. The lowest concentration of 25 and 50% recorded lowest percentage growth inhibition, whereas the highest concentrations of 75 and 100% recorded the highest percentage growth inhibition compared to control in all the interactions across the inoculation days (Figure 1,2,3, and 4).
DISCUSSION
An experiment was conducted to evaluate the effect of different plant extracts on one of the devastating fungal pathogen of tomato, A. solani. The study focused on how the plant extracts reduce or promote the mycelia growth and percent inhibition of the fungal pathogen under different concentrations of the extracts across the inoculation days (2-8 DAI). The result obtained from this study indicated that Alternaria solani was the causal agent for the tomato early blight. This is in line with several reports showing fungal pathogens causes spoilage in vegetables and fruits both in the field and storage (Olaitan, 2012; Oyewale, 2006), and they also suggest that the pathogen has the potential to cause severe damage to horticultural plants when the condition is favorable. Postharvest deterioration of fruits and vegetables is elevated due to present of fungal pathogens and favorable environmental conditions (Hong and Eum, 2020).
In this study, both the mycelia growth and percentage inhibition, significant differences existed among the plant extracts and the different concentration used in all the inoculation days (Table 1 and 6). Significant interactions were also observed between the plant extracts and concentrations in both mycelia growth and growth inhibition respectively.
The study shown that the lowest mean mycelial growth was observed on Neem in all the inoculation days. The result is similar to that of (Manasa et al., 2013; Koch et al., 2017; Munda et al., 2018) who attributed the activity of Neem extracts against different fungal pathogens due to the presence and abundance of bioactive compounds such as azadirachtin, that exhibits antifungal activities as well as the susceptibility of the pathogens to these compounds. Moreover, Ravi et al., (2017), also reported that Neem extracts inhibited the growth of fungal pathogens by lysing the fungal structures and inhibits its growth. Similarly, Neem oil was also found to be effective against some fungal species like Penicillium, Corticinium, Rhizoctonia, Aspergillus (Okigbo et al., 2018; Sinha et al., 2018). Wokocha and okekereke (2005) also reported that leaves extracts and Neem oil effectively inhibited the growth of Sclerotium rolfsii in-vitro, another devastating fungal pathogen of tomato.
In this study, after Neem, the second least mycelial growth was observed on PDA amended with Garlic extract. A similar study conducted by Rao et al., (2009), reveals that Garlic extracts and its oil significantly reduced mycelial growth of some fungal pathogens and possesses high anti-fungal and antioxidant ability. In conformity to our findings, recent reports on phytochemical analysis of Neem and Ginger by Gifoni et al., (2012) reported the antifungal inhibitory activity of Neem and Ginger against mycelial growth and spore germination of Fusarium solanim and A. solani at minimum inhibitory concentration (0.05 mg mL−1). However, in this report Neem extract ranked after Garlic and Ginger in reduction of the mycelial growth of A. solani the causal agent of tomato early blight.
Despite showing high mycelial growth by Ginger in this study, several studies reveled its antifungal effects against many fungal pathogens, due to the presence of bioactive compounds such as gingerols, geranial, and aromatic- curcumene among others (Munda et al., 2018).
The results on percentage growth inhibition indicates that Neem at 2-8 day after inoculation had higher percent inhibition followed by Garlic, while Ginger had the least percent inhibition compared to control and check respectively (Table 6). The results also shown that higher concentrations inhibits the growth of the fungus compared to lower concentrations. In a similar experiment by Batish et al., (2008), Neem and Garlic extract were found to be more effective in inhibition of fungal growth than Ginger rhizomes extract at 3, 6 and 9 DAI. The efficiency of these plant extracts to growth inhibition of the fungal pathogen could be attributed to the activities of azadirachtin compound in the Neem and aromatic phenols and many other compounds such as 1, 8-Cineole found in Garlic and Ginger which proved to have fungicidal properties.
CONCLUSION
Based on the results of this study, it was concluded that Neem and Garlic extracts contained more antifungal compound that suppress the growth of Alternaria solani, hence could be used as environmentally safe as well as affordable by the farmers for the management of early blight of tomato.
REFERENCES
Barnett H.L., Hunter, B.B. (2006). Illustrated genera of imperfect fungi. American Phytopathological Society (APS Press).
Batish D.R., Setia N., Singh H.P., Kohli R.K. (2008). Phytotoxicity of lemon-scented eucalypt oil and its potential use as bioherbicide. Crop Protection, 23: 1209-121.
Chohan S., R. Perveen, M.A. Mehmood, S. Naz, N. Akram (2015). Morpho-physiological studies, management and screening of tomato germplasm against Alternaria solani, the causal agent of tomato early blight. International Journal of Agric. Biology, 17: 111-118.
FAOSTAT (2023). Crops and livestock products [Online]. Food and Agricultural Organization of the United Nations. Available: http://www.fao.org/faostat/en/#data/TP [Accessed February 2024].
Fawole M.O., Oso B.A. (1995). Laboratory Manual of Microbiology. 1st edition. Spectrum Books Ltd, Ibadan, Nigeria. pp. 34‒35.
Gifoni J.M., Oliveira J.T., Oliveira H.D., Batista A.B., Pereira M.L., Gomes A.S., Oliveira H.P., Grangeiro T.B., Vasconcelos I.M. (2012). A novel chitin‐binding protein from Moringa oleifera seed with potential for plant disease control. Peptide Science, 98: 406-415.
Haruna S.G., Abdulkadir M.H., Adamu S.H., Lurwanu Y., Tijjani I. (2024). Biological based integrated management of fusarium wilt of tomato (fusarium oxysporum f. sp. lycopersici) using host resistance and trichoderma fortified compost. Nigerian Journal of Horticultural Science, 28: 64-76.
Hong S.J., Eum H.L. (2020). Determination of the harvest date and ripening phase of ‘Seolhyang’ Strawberry. Prot. Hortic. Plant Fact., 29: 62–72.
Koch W., Kukula-Koch W., Marzec Z., Kasperek E., Wyszogrodzka-Koma L., Szwerc W., Asakawa Y. (2017). Application of chromatographic and spectroscopic methods towards the quality assessment of ginger (Zingiber officinale) rhizomes from ecological plantations. International journal of molecular sciences, 18: 452.
Lurwanu Y., Wang Y.P., Wu E.J., He D.C., Waheed A., Nkurikiyimfura O., Wang Z., Shang L.P., Yang L.N., Zhan J. (2021). Increasing temperature elevates the variation and spatial differentiation of pesticide tolerance in a plant pathogen. Evolutionary Applications, 14: 1–12.
Manasa D, Srinivas P, Sowbhagya HB. (2013). Enzyme-assisted extraction of bioactive compounds from ginger (Zingiber officinale Rosc.). Food chemistry, 139: 509-514.
Muhammad A.S., Mohammed I.U., Ameh M., Bello I., Haliru B.S., Bagudo H.A., Sanda A. (2018). Isolation and identification of fungi associated with the spoilage of sweet orange (Citrus sinensis L.) and banana (Musa sapientum L.) in Sokoto Metropolis. Journal of Applied Biotechnology and Bioengineering, 5: 172‒182.
Munda S., Dutta S., Haldar S., Lal M. (2018). Chemical analysis and therapeutic uses of ginger (Zingiber officinale Rosc.) Essential Oil: A Review. Journal of Essential Oil Bearing Plants, 21: 994-1002.
OECD (2008). Consensus document on compositional considerations for new varieties of tomato: Key food and feed nutrients, toxicants and allergens, Series on the Safety of Novel Foods and Feeds, No. 17, OECD, Paris, www.oecd.org/env/ehs/biotrack/46815296.pdf
Okigbo R.N., Ezebo R.O., Ugwu S.C. (2018). Antifungal attributes of extracts of Ocimum gratissimum, Zingiber officinale, and Cymbopogon citratus on rot fungi of soursop fruit. Clinical Journal of Nutrition and Dietetics, 1: 1-7.
Olaitan, O.O. (2012). Bio-deterioration of sweet pepper in storage, inoculation induced quality changes, and control by modified atmosphere. Journal of Applied Sciences and Environmental Management, 16: 189-193.
Oyewale M. (2006). Fungal diseases of Bell pepper. http://acsconfex. com/acsgreeno6/technprogram/p26998.HTM (July 3, 2006).
Rao B.S.S., Shanbhoge R., Rao B.N., Adiga S.K., Upadhya D., Aithal B.K., Kumar M.R.S. (2009). Alcoholic extract of Cymbopogon citratus against radiation-induced DNA damage on V79 cells and free radical scavenging ability against radicals generated in vitro. Human Experimental Toxicology, 28: 195-202.
Ravi A., Varghese S., Edayileveetil Krishnankutty R. (2017). Biocontrol activity of the extract prepared from Zingiber zerumbet for the management of rhizome rot in Zingiber officinale caused by Pythium myriotylum. Archives of Phytopathology and Plant Protection, 50: 555- 567.
Sinha A., Singh S., Kumar S., Rai S. (2018). In vitro antifungal potency of plant extracts against post-harvest storage fungal pathogens of Zea mays L. International Journal of Current Microbiology and Applied Sciences, 7: 1236-1247.
Taofiq O. (2017). Trend analysis of tomato production in Nigeria (2010 – 2014). National Agricultural Extension and Research Liason Services, Ahmadu Bello University, Zaria, Kaduna State, Nigeria.
Vaughan J.G., C.A. Geissler (1997). The New Oxford Book of Food Plants, Oxford University press ltd, UK.
Workocha R.C., Okereke V.C. (2005). Fungitoxic activity on extracts of some medicinal plants on Sclerotium rolfsii, causal organisms of balsam stem rot diseases of tomato. Nigeria Journal Plant Protect., 22: 106-111.